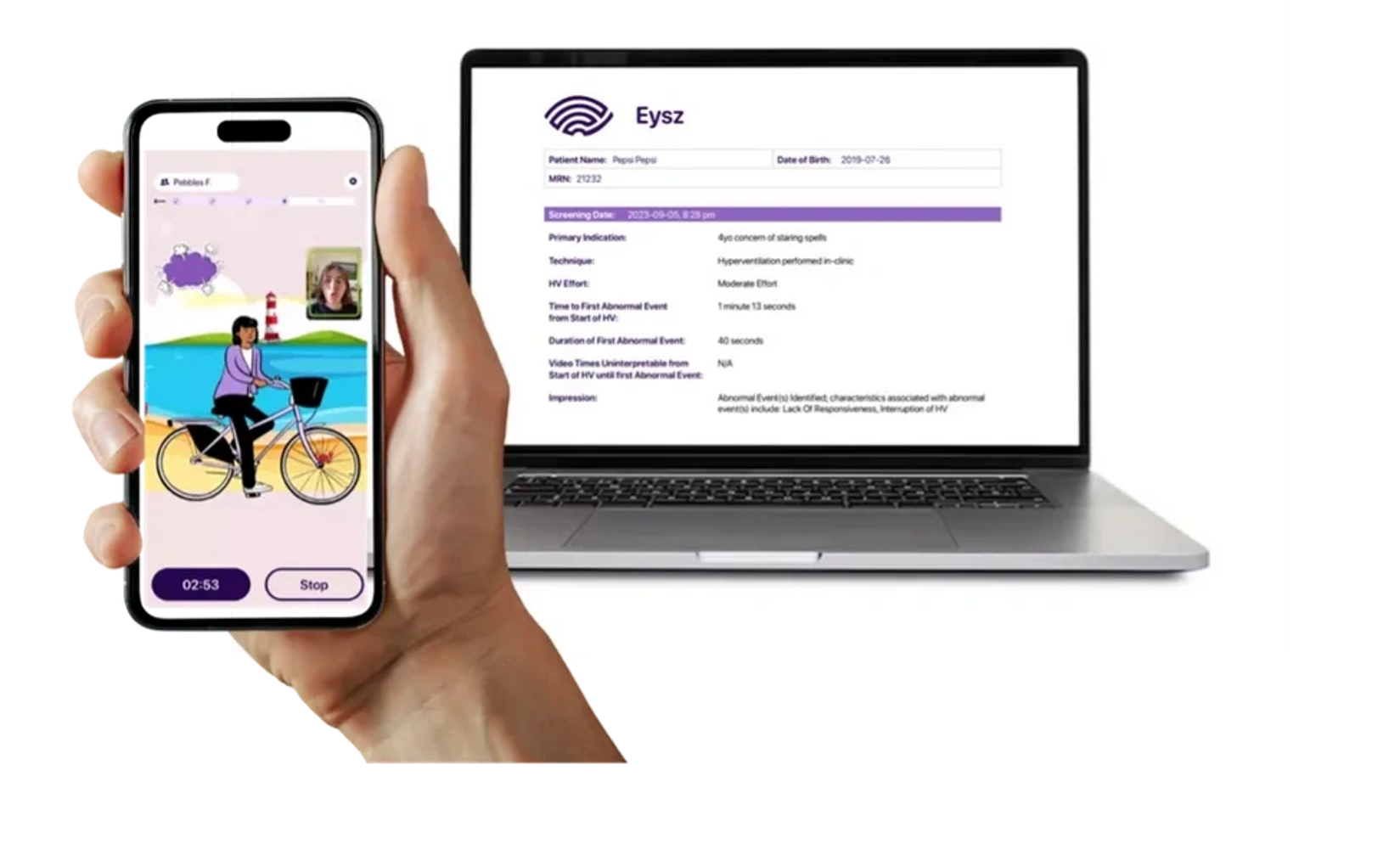
Differentiate ADHD and Childhood Absence Epilepsy (CAE), Confidently.
Eysz HyperventilationRecorder - the FDA Registered, gamified solution for objective in-office screening of absence seizures!

Eysz HyperventilationRecorder - the FDA Registered, gamified solution for objective in-office screening of absence seizures!


The American Academy of Pediatrics Guidelines on ADHD recommends to rule out alternative causes to inattention (such as absence seizures) before making a diagnosis of ADHD and to identify co-occuring conditions (such as absence seizures) that could impact ADHD treatment plans.

Clinical history and subjective screeners may not be enough to tell one condition from another. For example, 90% of parents of children with CAE report staring behaviors, as do 80% of parents of children with Inattentive ADHD.

Timing of conditions when they co-occur can make treatment planning a challenge. Up to 50% of children with CAE also have ADHD, with attention deficits presenting before the diagnosis of CAE.


